INTRODUCTION
Bryophytes are small, green group of surface-dwelling plants that make a significant contribution to the Himalayan biodiversity. These are highly specific group of plants with about 24,000 species distributed worldwide (Hallingbäck & Hodgetts, 2000), making them the second largest group of land plants after angiosperms (Alam, Sharma & Sharma, 2011). Liverworts and mosses are known to synthesize a greater range of secondary metabolites like terpenoids, alkaloids, glycosides, flavonoids, carotenoids, steroids, etc. Particularly, liverworts are well known as a rich source of sesquiterpenes and terpenoids with biological activities such as antifungal, antibacterial, antiviral, antioxidant and insecticidal activities (Subin et al., 2021). In recent years with the advancement of the GC-MS technology, discovery of the strong bioactive compounds of these plants have globally attracted the attention. GC-MS technique for analysing the phytochemical characterization and identification of new compounds with medicinal potential against diseases is considered to be the most appropriate and widely used method (Igwe et al., 2015). From the bryophytes, several bioactive substances have been extracted and identified, and they may serve as a source for future promising studies (Dziwak et al., 2022). Even though a single species is known to possess multiple compounds with multiple type of activities. Approximately, over 400 new compounds were isolated from bryophytes (Asakawa, 2007). In spite of the rich potential of Himalayan bryodiversity, very few liverworts and mosses were screened for their phyto-chemical characterization. Therefore, proper phytochemical screening of the potent bryophytes for chemical constituents and for isolating the novel bioactive compounds is of prime importance.
Keeping this in mind, a preliminary attempt was made to screen phytochemical characterization of a leafy liverwort P. asplenioides belonging to family Plagiochilaceae, flourishing well on the shady, moist, sloppy hill site in Kumaun region of the North-West Himalayas. Literature survey reveals that, there are no reports on the GC-MS analysis of a methanolic extract of the leafy liverwort P. asplenioides. However, few reports are available on the GC-MS analysis of the other bryophytes (Negi, Asthana & Chaturvedi, 2020; Negi, Tewari & Chaturvedi, 2018).
Materials and Methods
Collection of plant samples
The collection of P. asplenioides was made during the rainy season from the shady, moist, sloppy hill site of mixed oak forest at Mukteshwar (2000 m) region of Nainital. The liverwort population was found closely associated with mosses viz., Atrichum undulatum (Hedw.) P.Beauv., Brachythecium buchananii (Hook.) A.Jaeger and Thuidium cymbifolium (Dozy & Molk.) Dozy & Molk. The collected samples were brought to the laboratory in a sealed polyethylene bag. Pure population of a leafy liverwort was morphologically segregated and further examined under the microscope for proper identification. The voucher specimens are kept in laboratory for future reference.
Preparation of Plant Extract
The pure samples were washed thoroughly under running tap water to remove the impurities and was shade dried at room temperature. The samples were then crumbled into powder form by using an electric grinder. The powdered plant sample was extracted with methanol as a solvent for 24 hours 10gm/100ml accordingly, and was further shaken on a rotatory shaker at 280 rpm. The mixture was filtered with Whatman filter paper no. 1. Semi-solid extract was obtained after evaporating the filtrate and stored aseptically in an airtight container in the refrigerator for future use.
GC-MS (Gas Chromatography-Mass Spectrometry) Analysis
GC-MS analysis of sample extract was performed by using Trace 1300 GC Triplus RSH autosampler TSQ 8000 Evo Mass spectrometer (Thermo, USA) equipped with TG 5MS capillary column (30 m × 0.25 mm), 0.25 μm film thickness of stationary phase, 5% phenyl and 95% dimethylpolysiloxane). This was operated in split (split ratio 10:20) injection mode with an injector temperature of 80°C. Helium was used as a carrier gas with the flow rate of 1.0 mL min-1. The GC oven temperature was programmed as follows: 60°C (hold for 3 min), increased to 200°C at the rate of 8°C min-1 (hold for 5 min), increased to 230°C at the rate of 6°C (hold for 5 min) and finally reached to 290°C (hold for 20 min) at a rate of 10°C min-1. The total run time was 60 min. The ion source and mass interface temperature were set at 230°C and 300°C respectively. The mass spectrometer was operated at an electron energy of 70 eV. The samples were derivatized by using BSTFA as silylation agent. All the samples were analysed in full scan mode of mass in the range of m/z 50-800. 1µL of the sample was injected into a chromatographic system.
The mass spectrometer analyses the eluted compounds at different times revealing the nature as well as structure of the compounds. Interpretation of the mass spectrum of GC-MS was done with the help of a database of the National Institute of Standard and Technology (NIST). A comparison was made between the mass spectra of the unknown component and spectrum of the known component kept in the NIST Library. To determine the bioactivity of the selected substances, Duke phytochemical and ethnobotanical data was used.
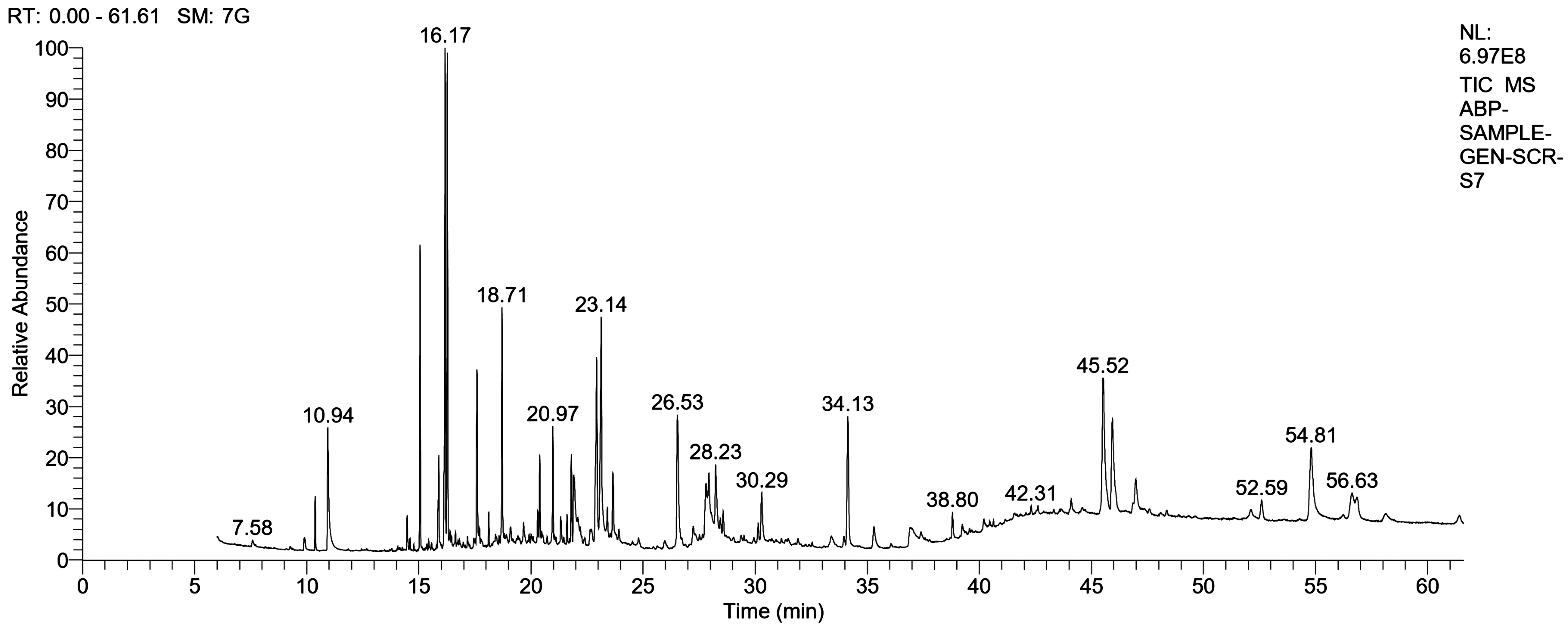
Figure 1. GC-MS Chromatogram of P. asplenioides methanolic extract
Results and Discussion
In the last few years, gas chromatography and mass spectrometry (GC-MS) has become firmly established as key technological platform for secondary metabolite profiling in plants (Kell et al., 2005). The chemical composition of the plant varies depending upon the choice of solvents used for the extraction procedure and the method used for preparing the extract. It is noteworthy that the difference in the chemical constituents of the plant extract may also be due to the ecological difference such as longitude, latitude, altitude, temperature, humidity, climate, soil, growth stage, collection time, etc.; different conditions influence the metabolic and biosynthetic pathways of the plant, therefore, numerous secondary metabolites will be synthesized under different environmental conditions (Daferera, Ziogas & Polissiou, 2000). The GC-MS chromatogram of P. asplenioides has shown the presence of sixteen different compounds (Table 1) represented by unique retention times and distinct peak areas (Fig. 1). The major components identified were (1,3,3,3-Tetrafluoro-2-trifluoromethyl-propenylsulfanylmethyl)-benzene (RT 7.58), Propella-2,4,7,9-tetraene (RT 10.94), Azulene (RT 16.17), Globulol (RT 18.71), Phytol (RT 20.97), L (+)-Ascorbic acid 2,6-dihexadecanoate (RT 23.14), Phytol (RT 26.53), 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol (RT 28.23), Eudesm-11-en-1à-ol (RT 30.29), Isolongifolol (RT 34.13 ), Rhodopin (RT 38.80), psi,psi-Carotene, 1,1’,2,2’-tetrahydro-1,1’-dimethoxy (RT 42.31), Ethyl iso-allocholate (RT 45.52), Pregn-5-en-20-one 3,16,17,21-tetrakis[(trimethylsilyl)oxy]-, O-(phenylmethyl) oxime (RT 52.59), 1,2-Bis (1-phenyl-3,6-diazahomoadamantantydene-9) hydrazine (RT 54.81), Cyano colchicine (RT 56.63). Out of total sixteen screened phytochemicals, five compounds namely, Azulene, Globulol, Phytol, L (+)-Ascorbic acid 2,6-dihexadecanoate and Ethyl iso-allocholate were turned out to be potentially bioactive compounds confirmed by Duke’s Phytochemical and Ethnobotanical database and other internet resources (Table 2). So, these bioactive compounds may be used in various pharmaceutical aspects. The effectiveness of these biologically active compounds will be one of the future objectives.
Table 1. Phytochemical characterization of P. asplenioides methanolic extract by GC-MS.
|
S. No.
|
R. Time
|
Molecular Formula
|
Molecular Weight
|
Compound Name
|
Structure
|
|
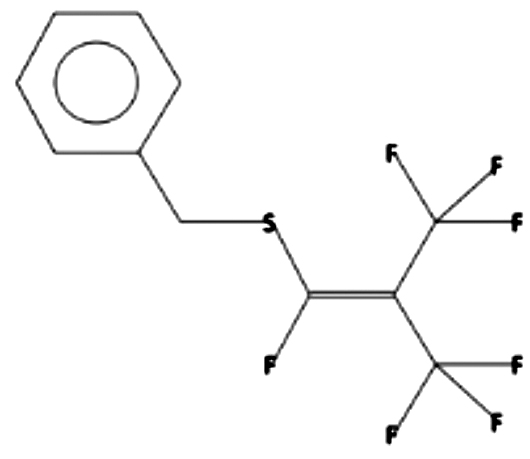
1
|
7.58
|
C₁₁H₇F₇S
|
304
|
(1,3,3,3-Tetrafluoro-2-trifluoromethyl-propenylsulfanylmethyl)-benzene
|
|
|
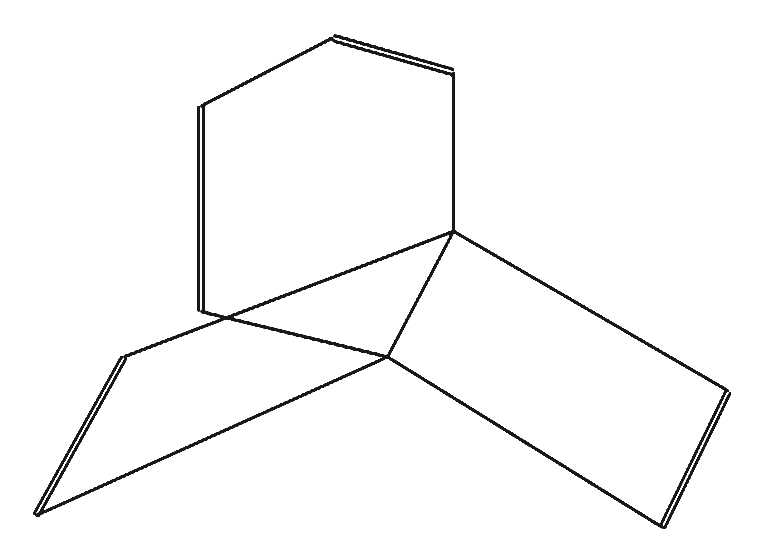
2
|
10.94
|
C₁₀H₈
|
128
|
Propella-2,4,7,9-tetraene
|
|
|
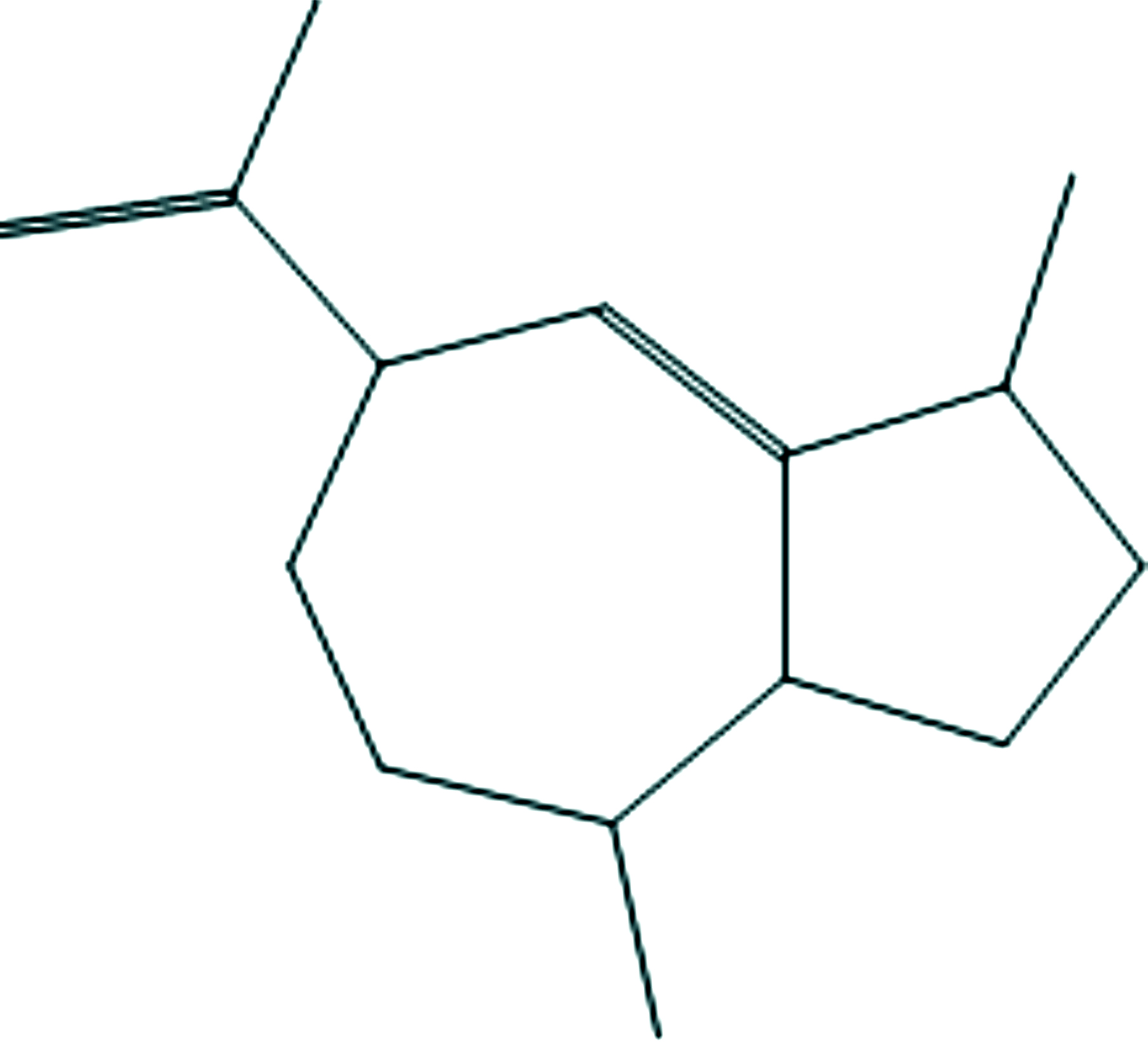
3
|
16.17
|
C₁₅H₂₄
|
204
|
Azulene
|
|
|
4
|
18.71
|
C₁₅H₂₆O
|
222
|
Globulol
|
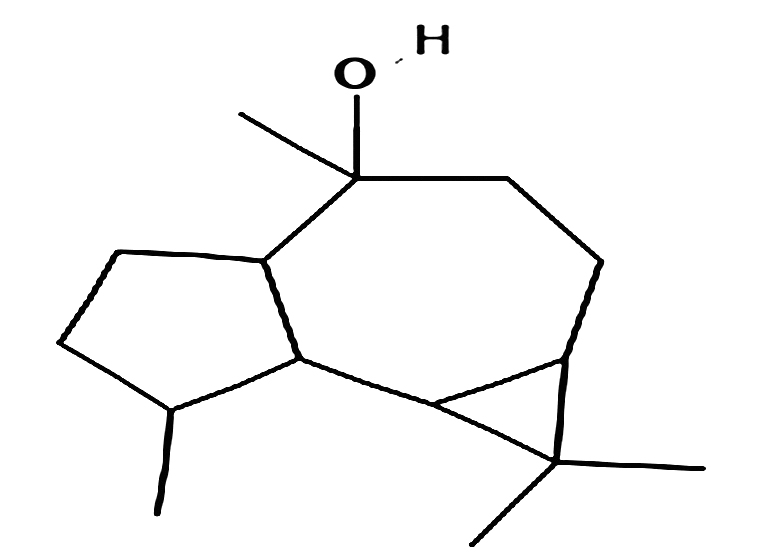
|
|
5
|
20.97
|
C₂₂H₄₂O₂
|
338
|
Phytol acetate
|
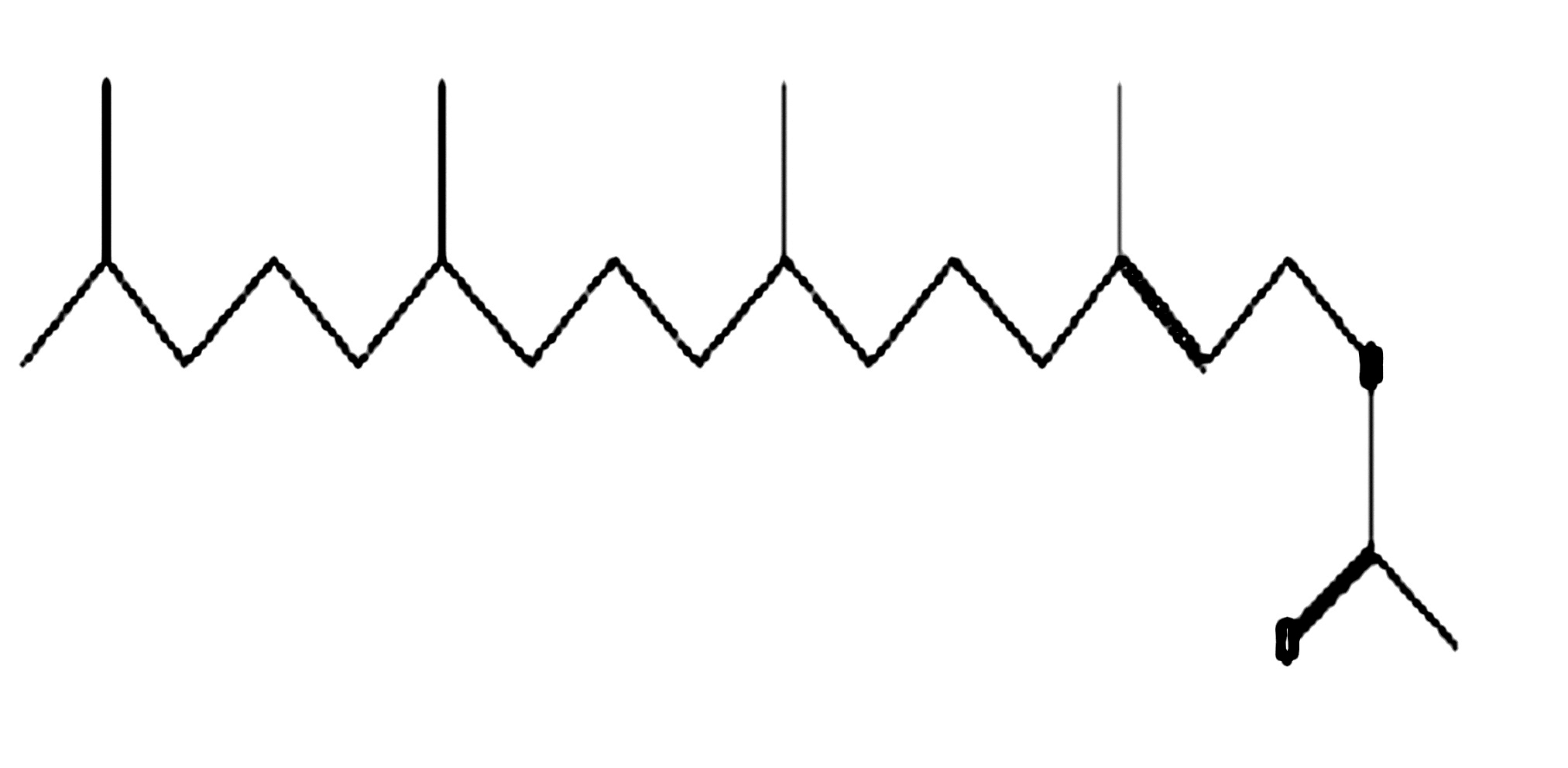
|
|
6
|
23.14
|
C₃₈H₆₈O₈
|
652
|
L (+)-Ascorbic acid 2,6-dihexadecanoate
|
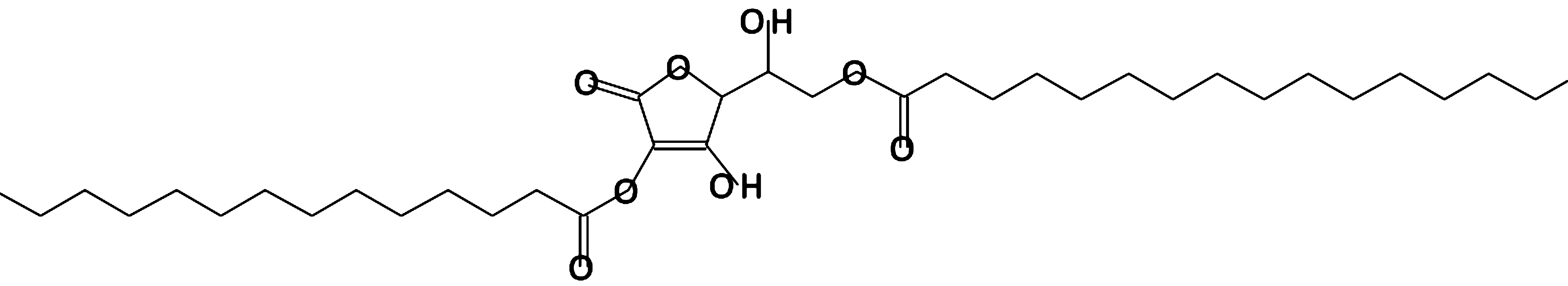
|
|
7
|
26.53
|
C₂₀H₄₀O
|
296
|
Phytol
|
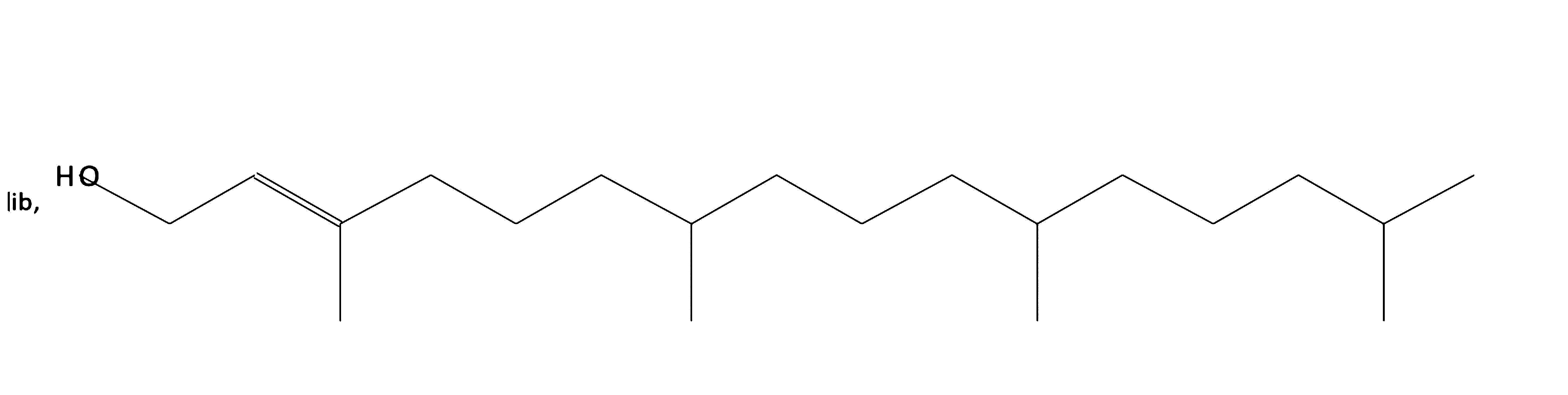
|
|
8
|
28.23
|
C₃₀H₅₂O
|
428
|
2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-
heptadeca-3,7,11,15-tetraenyl)-cyclohexanol
|
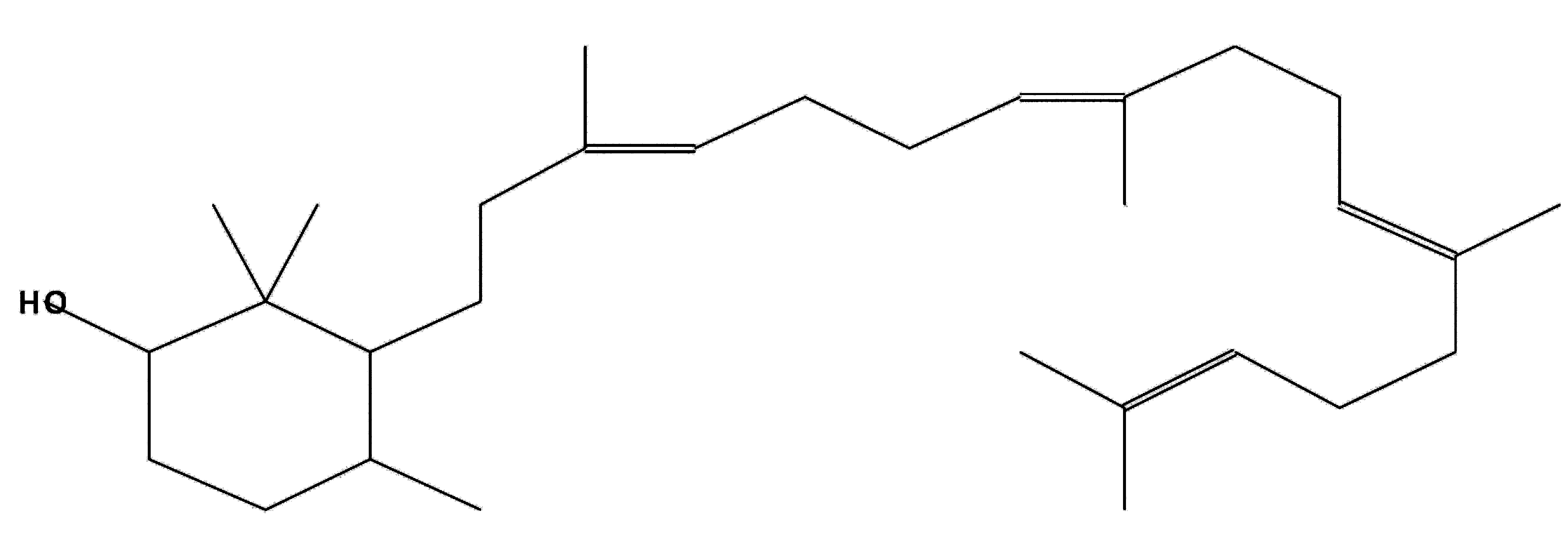
|
|
9
|
30.29
|
C₁₅H₂₆O
|
222
|
Eudesm-11-en-1à-ol
|
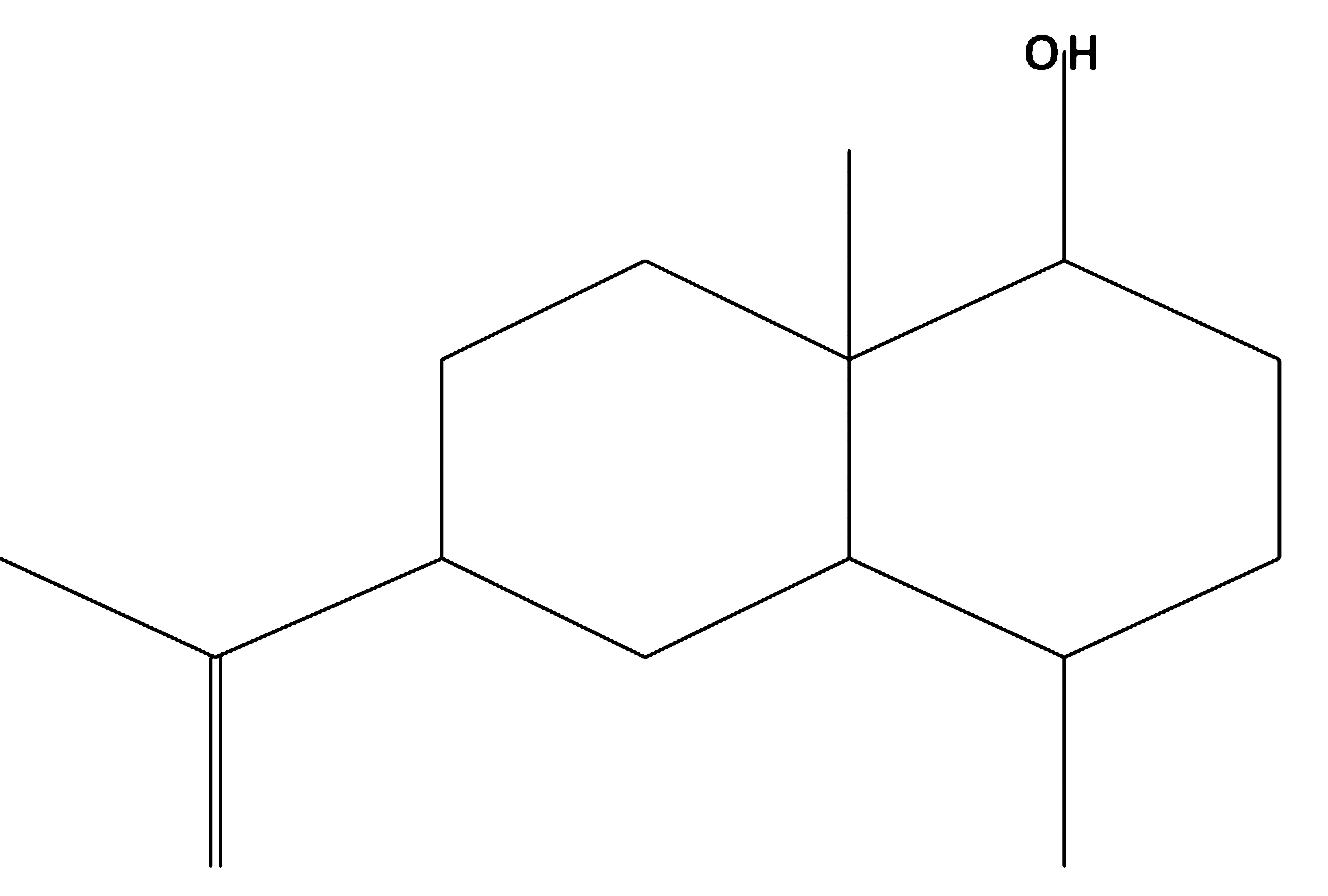
|
|
10
|
34.13
|
C₁₅H₂₆O
|
222
|
Isolongifolol
|
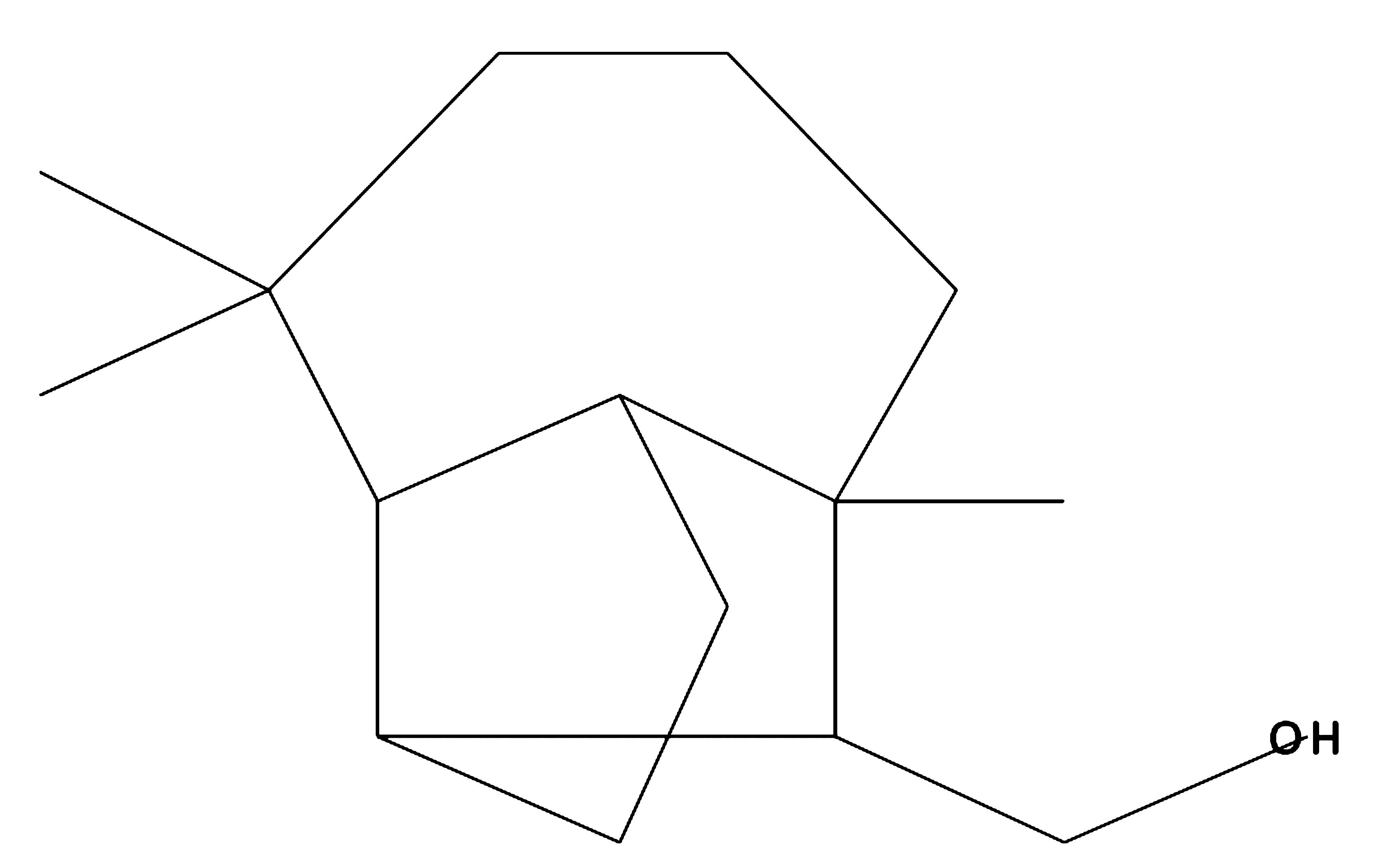
|
|
11
|
38.80
|
C₄₀H₅₈O
|
554
|
Rhodopin
|
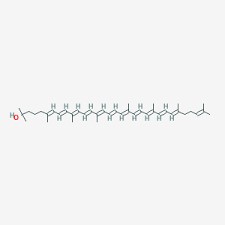
|
|
12
|
42.31
|
C₄₂H₆₄O₂
|
600
|
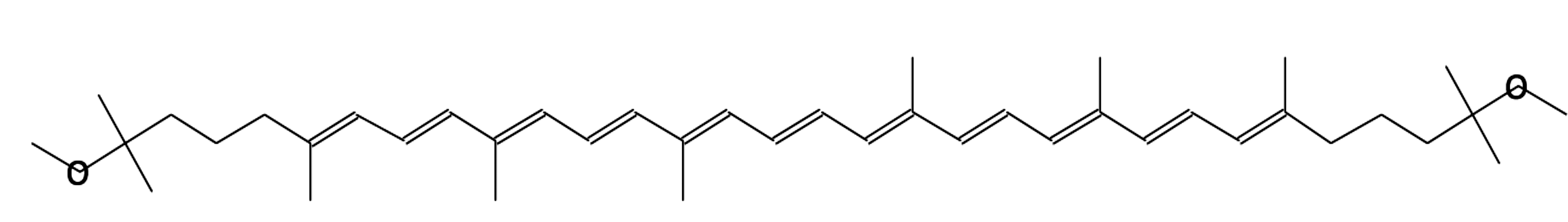
psi,psi-Carotene, 1,1',2,2'-tetrahydro-
1,1'-dimethoxy
|
|
|
13
|
45.52
|
C₂₆H₄₄O₅
|
436
|
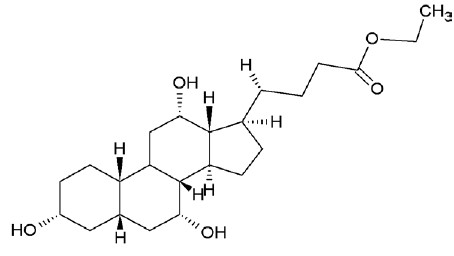
Ethyl iso-allocholate
|
|
|
14
|
52.59
|
C₄₀H₇₁NO₅Si₄
|
757
|
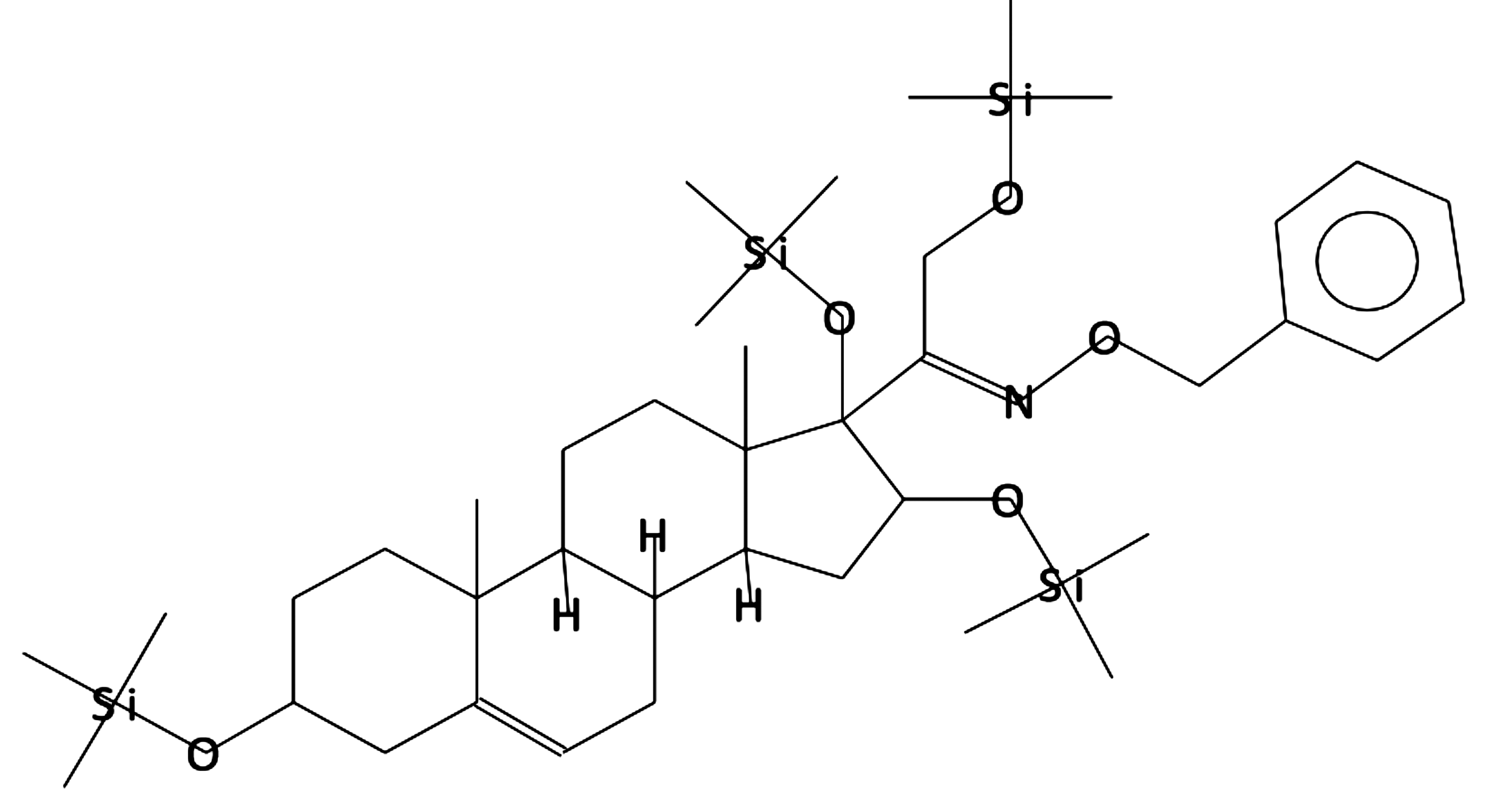
Pregn-5-en-20-one, 3,16,17,21-tetrakis
[(trimethylsilyl)oxy]-, O-(phenylmethyl)
oxime
|
|
|
15
|
54.81
|
C₃₀H₃₆N₆
|
480
|
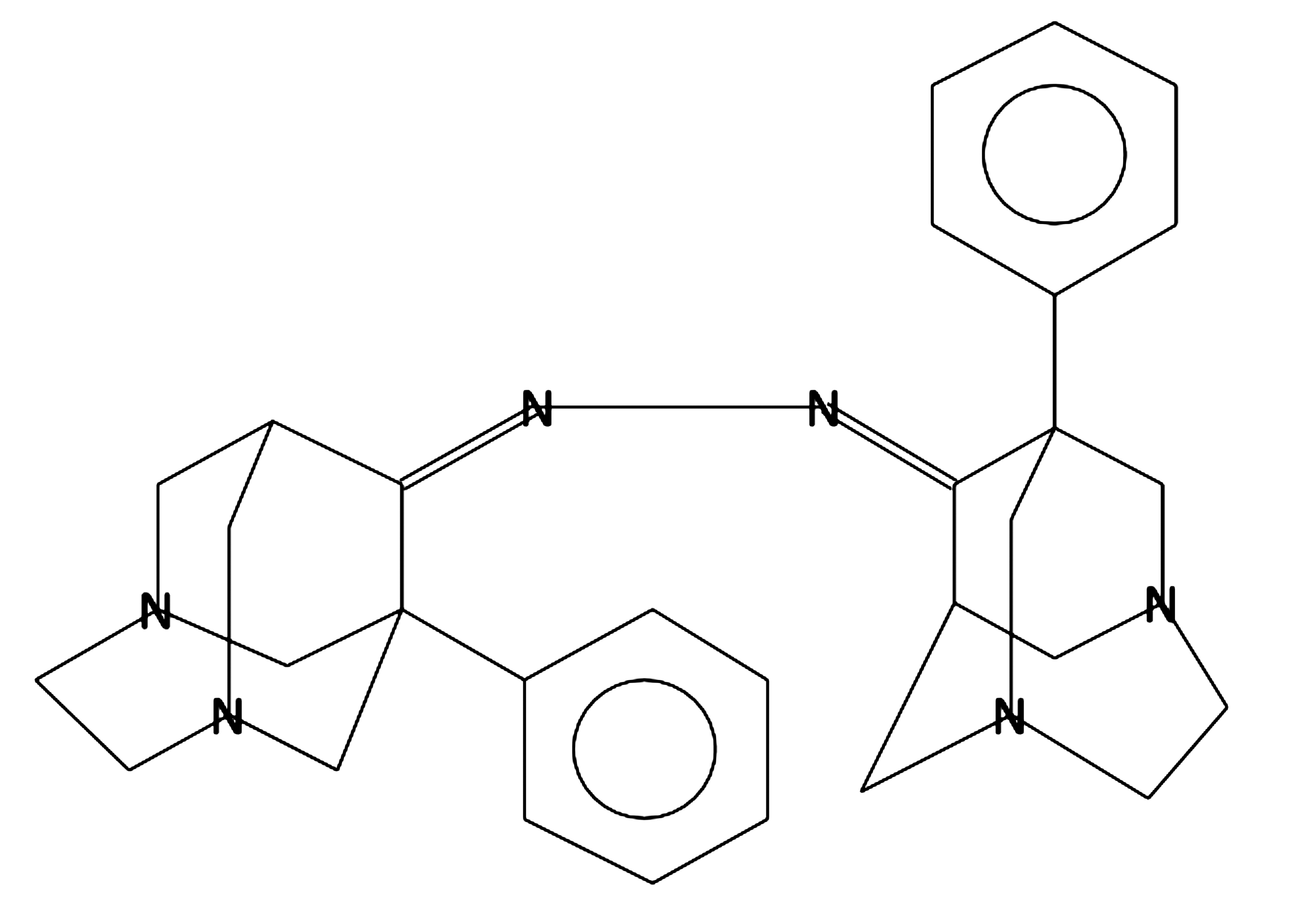
1,2-Bis
(1-phenyl-3,6-diazahomoadamantantydene-9) hydrazine
|
|
|
16
|
56.63
|
C₂₃H₂₄N₂O₆
|
424
|
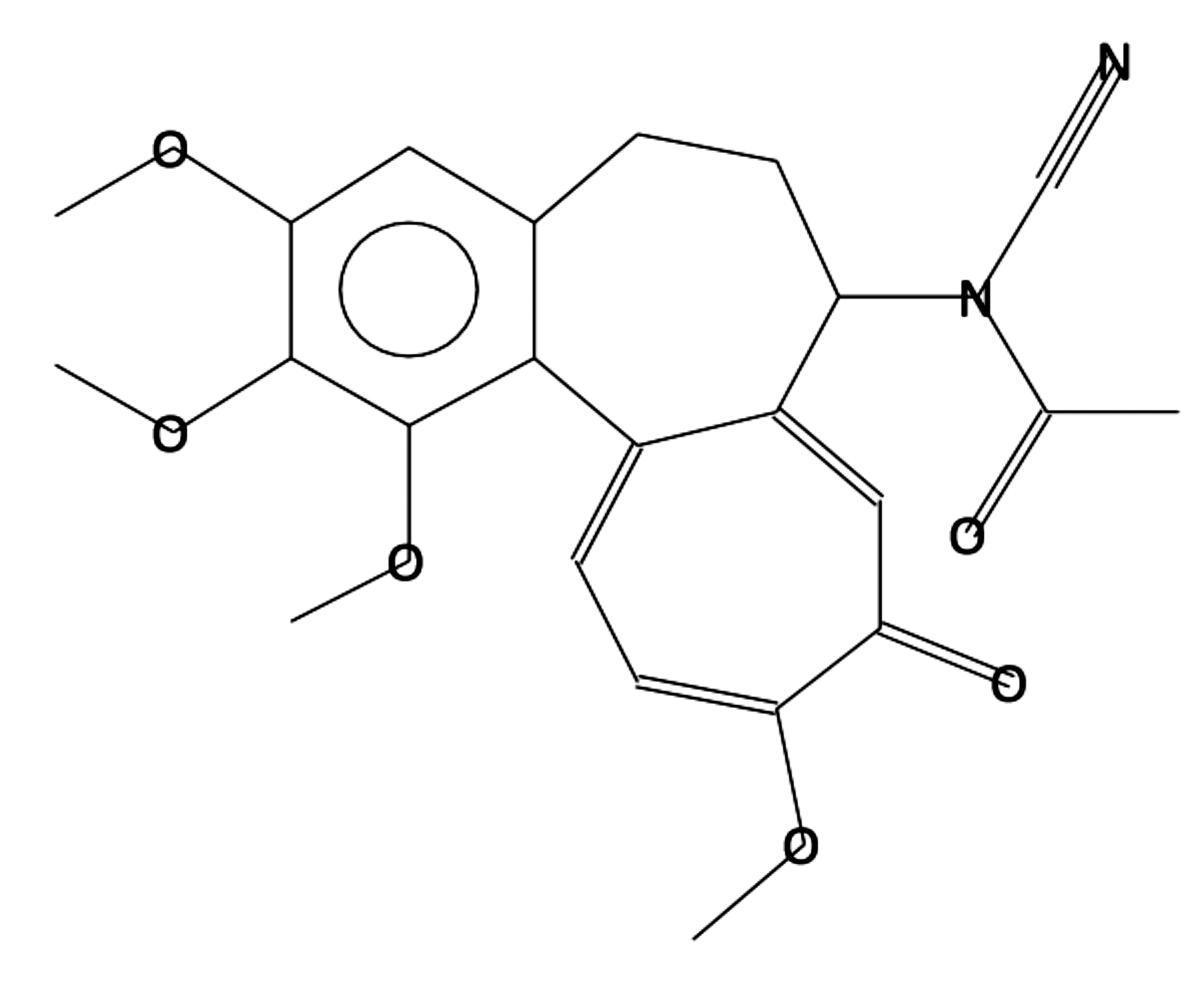
Cyano colchicine
|
|
Table 2. Biological Activity of Phyto components identified in the methanolic extract of leafy liverwort P. asplenioides
|
S. No.
|
Compound Name
|
Biological Activity
|
|
1.
|
Azulene
|
Antimicrobial, Antihelmintic, Antifeedant, Anti-inflammatory
|
|
2.
|
Globulol
|
Antimicrobial Activity (Tan et al., 2008)
|
|
3.
|
L (+)-Ascorbic acid 2,6-dihexadecanoate
|
Antimicrobial Activity (Karthikeyan et al., 2014)
|
|
4.
|
Phytol
|
Antimicrobial, Antioxidant, Anti-inflammatory, Anti-allergic (Ghaneian et al., 2015)
|
|
5.
|
Ethyl iso allocholate
|
Anti-microbial, Diuretic, Anti-inflammatory, Antiasthma Activity
(Muthulakshmi, Jothibai & Mohan, 2012)
|
Conclusion
The findings of the present investigation suggest that methanolic extract of P. asplenioides was suitable to identify the various phytochemical compounds present in the plant. The crude extract of the studied plant also showed significant biologically active compounds, this indicates the great phytochemical potential of the Himalayan bryophytes. Thus, in the future, more bryophytes, liverworts and mosses should be screened so that bryophytes with active biological potential can be documented. The current work is the first attempt to screen the phytochemical constituents of the Himalayan leafy liverwort P. asplenioides. Based on the preliminary results obtained future chemical and pharmacological investigations may be performed to elute the novel bioactive compounds from them which may be useful for the treatment of many diseases. The results obtained indicate that P. asplenioides could be regarded as a plant of phytopharmaceutical significance.
Acknowledgments
The authors are thankful to the Jawaharlal Nehru Memorial Fund, New Delhi for providing financial support. We are also grateful to the Principal and Dr Saraswati Bisht, Head of the Department of Botany, Indira Priyadarshini Government Girls Post Graduate College of Commerce, Haldwani for providing laboratory facilities. We also express our gratitude to Dr A.B. Pant, Senior Principal Scientist, CSIR - Indian Institute of Toxicology Research and Dr Satya Narayana for their support in analysing the sample. Thanks, are also due to the Research Scholars of the Bryology Laboratory for their help.















