INTRODUCTION
The Himalayan region characterized by young geological formations such as geomorphological processes, heavy rainfall and sedimentary rocks are among the most fragile areas. In addition to natural factors, human interventions especially broadening of existing roads, mining, deforestation and urbanization have worsened the situation. Landslides are particularly common in lower middle hills located in close proximity to two major tectonic discontinuities, the Main Boundary Thrust (MBT) and the Main Central Thrust (MCT). The state of Uttarakhand has a history of unprecedented catastrophes and several landslides due to heavy rainfall in the past. In August 1998, 103 people were killed in landslides that hit Madhmaheshwar and the Kali Ganga valley. In 2001, about 20 people were killed in landslides in Phata and Gad areas. About 16 people were killed in another landslide on 5 July, 2004 which occurred in Badrinath (NIDM, 2015). Almost every monsoon season landslide occurs on mountain roads of about 400-700 cubic meters/km. Between 1960 and 1990, annual damage from landslides was estimated $1,500 million and annual deaths were 1,000 (Pandey et al., 1999). Based on the overall experience of landslides, rough estimates of the loss to the Indian economy are in the order of Rs. 250-300 crores per year, for the whole country (Joshi, 2013).
Several efforts have been made to stabilize the hill slopes in Uttarakhand using cover crops, plantation of fast growing natural and exotic species. However, success of slope stabilization depends on presence of soil mycorrhizae in the area. These fungi are very successful inhabitants of soil, due to their high plasticity and their capacity to adopt various forms in response to adverse or unfavourable conditions (Sun et al., 2005). AM associations are known as arbuscular mycorrhizas, vesicular arbuscular mycorrhizas and are abbreviated as AM/VAM (Smith, 1994; Walker, 1995). VAM association involves fungi in the Zygomycete order Glomales and the roots of a wide diversity of plants (Bécard & Pfeffer, 1993). More than 80 per cent of the plant species in nature form a symbiotic relationship with the beneficial mycorrhizal fungi (Chadwick, 2014).
Studies on arbuscular mycorrhizae of plants have been continuously from the time of discovery of this phenomenon (Frank, 1985). Mycorrhizal fungi bind the soil and also promote the formation of soil structure, allowing movement of air and water through the rooting volume rather than across the surface (John, 2000). Arbuscular mycorrhizal symbiosis is a mutually beneficial interaction between fungi and land plants and promotes global phosphate cycling in terrestrial ecosystems (Tanaka, 2022). AMF colonization in pioneer roots indicated association of mycorrhizal fungi is needed to help plant survival and improve the soil properties. Furthermore, landslide impacted areas can be restored and used as agriculture areas (Prayudyaningsih et al., 2021). This paper deals with diversity of arbuscular mycorrhizae in landslide areas.
Study Area
This study was conducted in six of the seven districts of the Garhwal region in Uttarakhand. Samples were taken for large landslides and highways, state highways, and county roads. Landslides that lasted more than 5-10 years and caused serious damage to ecology and blocked roads for a longer period were mainly selected for the study. An extensive survey was carried out for sample collection on NH 58 (now NH 07), NH 107, NH 34, NH 507, NH 709B, NH 134, NH 545 and various state and territory highways of Garhwal region. A total of 20 large landslides have been identified between altitude of 687 m (Sirobagarh) to 2290 m (DM landslide).
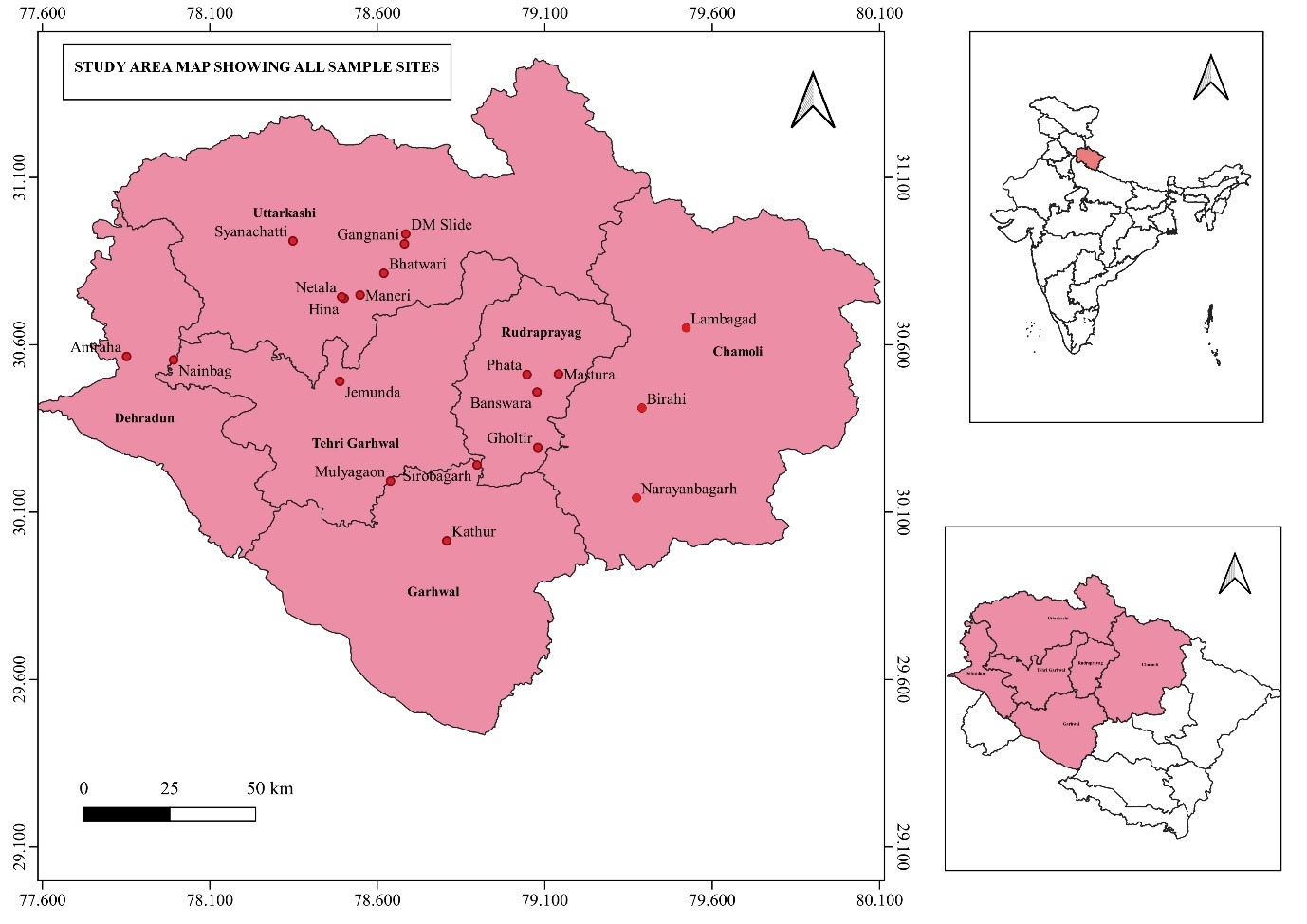
Figure 1. Study area map showing all sampled landslides.
Methodology
Analysis of soil and root samples
Samples (fine roots and rhizosphere soil) were randomly collected from landslide areas. At least six soil samples per landslide during different seasons weighing 200 g soil was collected. The soil samples were air-dried in the shade (at room temperature) for about 15 days. 10 g soil from each sample was used to isolate VAM/AM fungal spores and at least 20 samples per landslide were examined. The root samples were washed with tap water and kept in a dry and cool place for examination.
VAM/AM fungal species were isolated from soil samples according to the wet sieving and decantation techniques of Gerdemann and Nicolson (1963) and identified using the standard monograph of Shenck and Pérez (1990) and the INVAM website. Root clearing and staining were performed according to the procedures described by Philips and Hayman (1970). The percentage of mycorrhizal colonization was calculated using the following formula:
Per cent mycorrhizal = Number of segments colonized × 100
colonization Total number of segments observed
Statistical analysis
Statistical analysis of VAM/AM fungal spores in soil samples, Fisher’s test is applied using Minitab 18 software.
Various parameters such as frequency (FO), relative abundance (RA), importance (IV), spore density (SD), species richness (SR), Shannon-Wiener index (H) and evenness (E) were calculated using the following formulas:
Number of samples in which the
FO = species or genus were observed × 100
Total samples
RA = Spore number of species or genus × 100
Total spore number
IV = (FO+RA)/2
SD = Spore number
100 gm air-dried soil
SR = Species number
soil sample
k
H = ∑ (Pi ln Pi); Pi = ni/N
i=1
where ni is the spore number of a species and N is the total number of identified spore samples.
E=H/Hmax; Hmax= ln S, where S is the total number of identified species.
Results
VAM/AM fungi
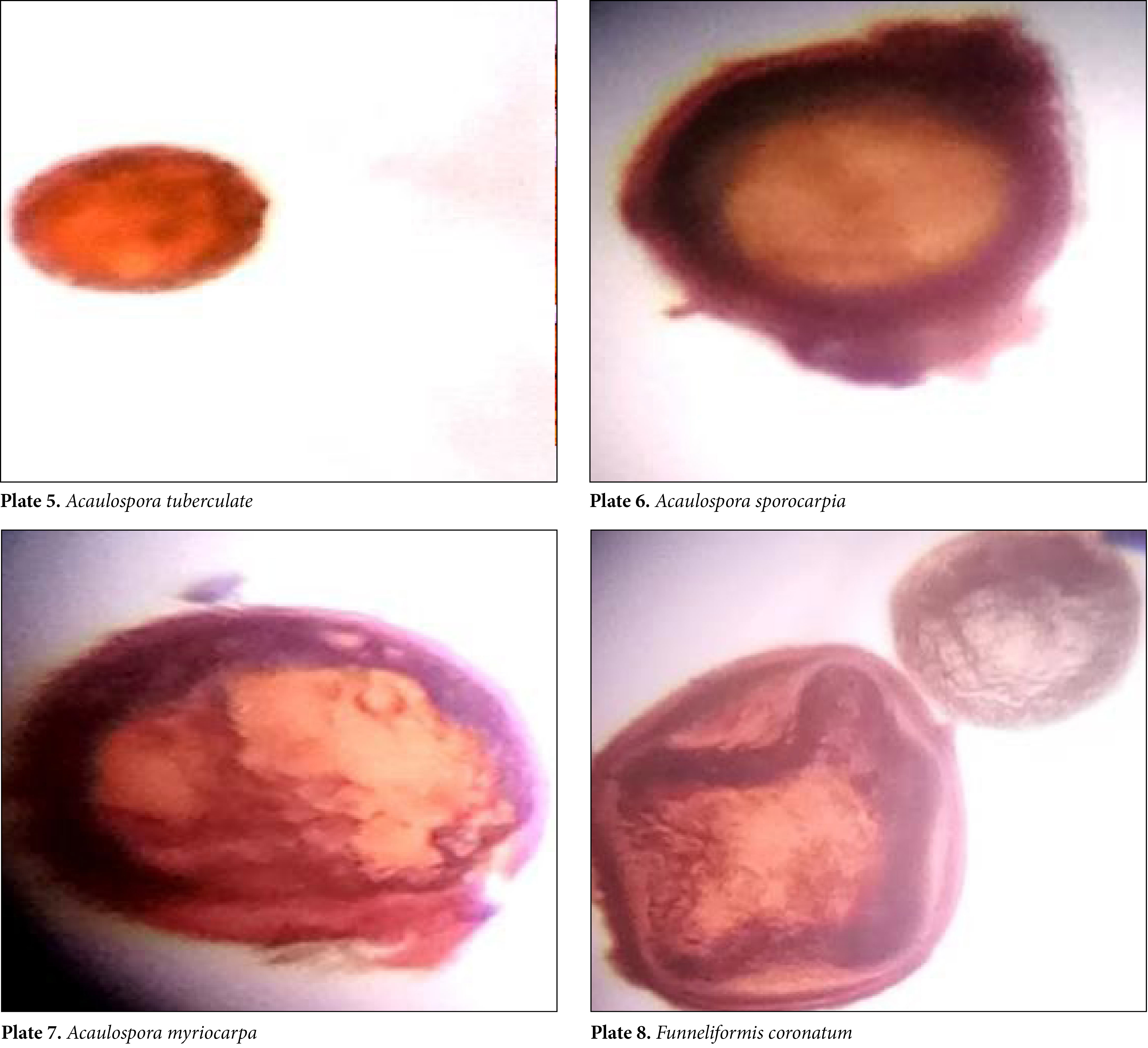
After examining 720 soil samples from 20 landslides, 8 mycorrhizal species from 2 genera (Acaulospora and Glomus) were found dominant. Glomus macrocarpum, Glomus fasciculatum, Funneliformis coronatum, Acaulospora laevis, Acaulospora foveata, Acaulospora tuberculata, Acaulospora sporocarpia and Acaulospora myriocarpa are the mycorrhizal species found in most of the landslides of Garhwal region. But the spore count per 10 g of soil is very less in comparison to other undisturbed soils.
A total of 62.5% Acaulosporaceae and 37.5% Glomeraceae AMF spores were identified. Among the mycorrhizal species mentioned above, Glomus fasciculatum and Acaulospora laevis were found in 3 districts and Glomus macrocarpum in 2 districts. The remaining species were found in only one of each region. Based on the prevalence and the number of spores found in 720 soil samples, it was revealed that Glomus macrocarpum (13 spores/100 g soil) is the dominant species in spore density, followed by Glomus fasciculatum (11 spores/100 g soil) and Acaulospora laevis (9 spores per 100 g soil) respectively.
Mean value calculated by One-way ANOVA at significant level (p<0.05) revealed that Glomus macrocarpum has the highest mean value (1.317) followed by Glomus fasciculatum (1.133) and Acaulospora laevis (0.925) while Acaulospora sporocarpia has the lowest (0.1250) (Table 1). Mean value is showing the presence of different mycorrhizal species in the tested soil samples at the 95% confidence interval.
Table 1. Mean value of mycorrhizal spores.
|
Species
|
Mean
|
Grouping
|
|
Glomus macrocarpum
|
1.317
|
A
|
|
Glomus fasciculatum
|
1.133
|
A
|
|
Acaulospora laevis
|
0.925
|
A
|
|
Acaulospora myriocarpa
|
0.1833
|
B
|
|
Acaulospora tuberculata
|
0.1667
|
B
|
|
Acaulospora foveata
|
0.1583
|
B
|
|
Funneliformis coronatum
|
0.1417
|
B
|
|
Acaulospora sporocarpia
|
0.1250
|
B
|
Grouping is showing the highest presence of mycorrhizal species in the rhizosphere soil of all the tested soil samples at the significance level (p<0.05). The maximum significant present mycorrhizal species were grouped in group A, while less significance was grouped in group B.
Interval plot of mycorrhiza in soil, justifying the mean value and grouping of mycorrhizal species present in the tested soil samples. (Fig. 2)
After analyzing the Individual tests for difference of means at the 95% confidence interval revealed that A. laevis- A. myriocarpa, A. laevis- A. tuberculata, A. laevis- A. sporocarpia and A. laevis- A. foveata showed positive mean difference, while, A. myriocarpa- G. marcrocarpum, A. tuberculata- G. marcrocarpum, A. sporocarpia- G. marcrocarpum, A. foveata- G. marcrocarpum, F. coronatum- G. marcrocarpum, A. myriocarpa- G. fasciculatum, A. tuberculata- G. fasciculatum, A. sporocarpia- G. fasciculatum, A. foveata- G. fasciculatum, F. coronatum- G. fasciculatum and F. coronatum- A. laevis showed negative mean difference. Rest of the combinations showed no difference in mean difference (Fig. 3).
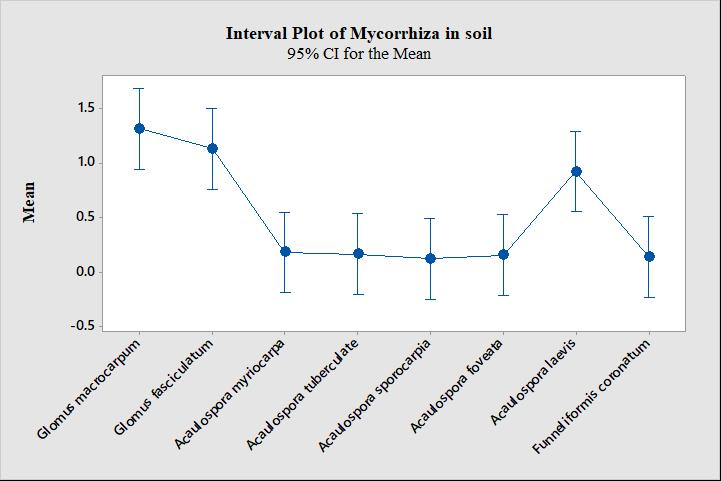
Figure 2. Interval plot of mycorrhiza in soil.
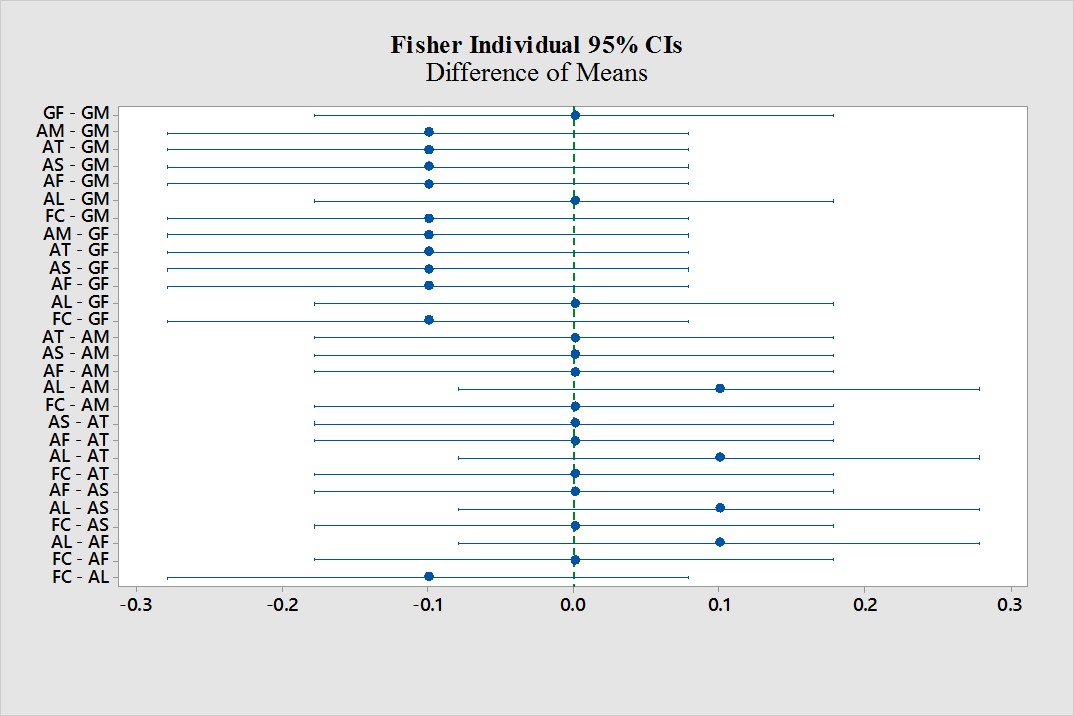
Figure 3. Difference of Means.
AMF Community Composition and Diversity
Mycorrhizal spores in rhizosphere soils of different plant species were studied for frequency of occurrence (%), relative abundance (%), importance value (%). Analysis showed that G. macrocarpum, G. fasciculatum and A. laevis have the highest FO percentage (42.86%) in comparison to other mycorrhizal spores (14.29%). G. macrocarpum (0.32%) has the highest RA percentage among all the spores while A. sporocarpia (0.03%) and F. coronatum (0.03%) showed lowest RA percentage. With 21.59%, G. macrocarpum has highest IV percentage followed by G. fasciculatum (21.58%) and A. laevis (21.56%) while rest of all species have the same IV percentage (7.16%) (Table 2).
Table 2. Frequency of Occurrence (FO), Relative abundance (RA) and Importance value (IV) of AM fungi.
|
Tree Species
|
DB
|
RS
|
EA
|
CD
|
WF
|
DV
|
DT
|
FO%
|
RA%
|
IV%
|
|
Mycorrhiza spp.
|
|
|
|
|
|
|
|
|
|
|
|
Glomus macrocarpum
|
-
|
*
|
-
|
-
|
-
|
*
|
*
|
42.86
|
0.32
|
21.59
|
|
Glomus fasciculatum
|
-
|
-
|
*
|
-
|
*
|
-
|
*
|
42.86
|
0.31
|
21.58
|
|
Acaulospora myriocarpa
|
*
|
-
|
-
|
-
|
-
|
-
|
-
|
14.29
|
0.04
|
7.16
|
|
Acaulospora tuberculata
|
-
|
-
|
-
|
-
|
*
|
*
|
-
|
14.29
|
0.04
|
7.16
|
|
Acaulospora sporocarpia
|
-
|
-
|
-
|
-
|
*
|
-
|
-
|
14.29
|
0.03
|
7.16
|
|
Acaulospora foveata
|
-
|
-
|
-
|
*
|
-
|
-
|
-
|
14.29
|
0.04
|
7.16
|
|
Acaulospora laevis
|
-
|
*
|
*
|
*
|
-
|
-
|
*
|
42.86
|
0.27
|
21.56
|
|
Funneliformis coronatum
|
*
|
-
|
-
|
-
|
-
|
-
|
-
|
14.29
|
0.03
|
7.16
|
|
SR
|
2
|
2
|
2
|
2
|
3
|
2
|
3
|
|
|
|
DB= Desmostachya bipinnata, RS= Rumax hastatus, EA= Eupatorium adenophorum, CD= Cynodon dectylon, WF=Woodfordia fruticosa, DV= Debregeasia velutina, DT= Desmodium triflorum.
SR of AM fungi in rhizosphere soil of D. velutina (0.22±0.08) has significantly higher than that of E. adenophorum (0.02±0.01). The H value of D. velutina (0.09±0.03) has the highest and lowest value is of E. adenophorum (0.36±0.13). E value of D. velutina (0.52±0.18) has the significantly higher than that of E. adenophorum (0.13±0.05) (Table 3).
Table 3. Diversity indices of AM fungi present in rhizosphere soil of different plants.
|
|
DB
|
RS
|
EA
|
CD
|
WF
|
DV
|
DT
|
|
SR
|
0.05±0.02
|
0.07±0.03
|
0.02±0.01
|
0.05±0.02
|
0.05±0.02
|
0.22±0.08
|
0.04±0.01
|
|
H
|
0.23±0.08
|
0.19±0.07
|
0.36±0.13
|
0.24±0.08
|
0.30±0.11
|
0.09±0.03
|
0.33±0.12
|
|
E
|
0.34±0.12
|
0.28±0.10
|
0.52±0.18
|
0.35±0.12
|
0.28±0.10
|
0.13±0.05
|
0.30±0.11
|
DB= Desmostachya bipinnata, RS= Rumax hastatus, EA= Eupatorium adenophorum, CD= Cynodon dectylon, WF=Woodfordia fruticosa, DV= Debregeasia velutina, DT= Desmodium triflorum, SR= Species richness, H= Shannon- Wiener index, E= Evenness
Discussion
The present study showed that genus Acaulospora has the highest number of spores, followed by genus Glomus while Glomus macrocarpum has highest spore density in landslide areas. Study by Wang et al. (2019) in a planted forest in eastern China, showed that Glomus had the highest number of AMF spores, followed by Rhizophagus. Prayudyaningsih et al. (2021) found that the genus Acaulospora had highest spore density followed by Glomus. Błaszkowski (1993a,b); Kowalczyk and Błaszkowski, (2011); Jamiołkowska et al. (2018) showed in their study that fungi of the genus Glomus are common in agricultural lands. Spores belonging to the genera Gigospora and Sclerocytis are more common in uncultivated soil. Khalil, Loynachan and McNabb Jr. (1992); Oehl et al. (2005) showed in their study on endomycorrhizal fungal communities, that the level of plant root colonization is generally not correlated with the spore population in the soil. The distribution of spores of mycorrhizal fungi in the soil profile depends on the degree of penetration of the roots of the host plant into the soil. Singh, Tiwari and Dkhar (2003) in their study on VAM species diversity in soils of cultivated clearing and natural forests of Arunachal Pradesh, showed that jhum clearing contained less VAM fungal population and less number of species than natural forest. The maximum number of VAM fungi were Glomus and Acaulospora.
Khalil and Loynachan (1994) concluded in their study that topographic field location (e.g. slope, valley) can also affect the AMF spore population, which is related to the AMF spore nutrient concentration and water migration. The present study supports their finding that the highest spore density of 13 spores/100 g soil was observed, which is very less compared to the undisturbed soil. Presence of mycorrhizal fungi is beneficial for the establishment of the plants in the disturbed sites as these fungi are known to absorb water and minerals from the soil.
Highest FO value of examined rhizosphere of herbs and shrubs of present study is lower and lowest FO value is higher than the study conducted by Wang et al. (2019) in rhizosphere of planted tree species, while this pattern can also be seen in RA and IV value. Prayudyaningsih et al. (2021) in their study also revealed that around 30 pioneer plant species were found colonized, most of which were herb and grass species.
The SR value of AMF in rhizosphere soil varies according to the host plant, micro-environment and different other factors. Present study revealed that W. fruticosa and D. triflorum have maximum number of spores in their rhizosphere soil and SR value was highest for D. velutina.

Conclusion
This study reveals that the presence of AM fungi was very less in comparison to the undisturbed sites. Total eight AM fungi were isolated from the soil samples in which Acaulospora was the dominant genera followed by Glomus. While Glomus has the highest spore density in the rhizosphere soil of different plants. It is recommended that both the genera could be introduced for the restoration of landslide areas by afforestation.





