INTRODUCTION
Jute fibre is biodegradable natural fibres which successfully replaced synthetic fibre composites and glass fibres. Jute is grown in tropical countries and is one of the strongest fibre with low cost. Jute fibre composite has several attractive advantages over synthetic and glass fibres, like low processing cost, low density, stiffness and excellent mechanical properties. These advantages make jute a very attractive reinforced fibre for composites and increased attention in construction, automotive, aerospace and many other industries (Farzana et al., 2022).
Natural fibres are eco-friendly, cost-effective, lightweight, renewable, have better thermal properties and corrosion resistance capabilities (Song et al., 2021).
Composites are materials formed by aligning extremely strong and stiff continuous or chopped fibres in a polymer resin matrix or binder. The fibres are typically 50 times stronger and 20-25 times stiffer than the matrix polymers. The role of matrix is primarily that of glue or binder which enables the fibres to support the applied load. Fibres are combined with resin and laminated to support the applied load. Many laminates exhibit mechanical properties equal to or exceeding those of most metals. Combining and orienting materials to achieve superior properties is an old and well-proven concept; examples of this synergism are around in nature. Wood contains an oriented hard phase which provides strength and stiffness. Other natural composites are found in teeth, bones, bird feathers and plant leaves. The use of chopped straw by the Israelites to control residual cracking in bricks is one example. More representative of modern structural composites are Mongolian bows, which are laminates of wood, animal tendons and silk (Gordon, 1976).
In recent years, there has been increasing interest in natural fibres as a substitute for glass fibres mainly because of their low specific gravity, low cost, as well as their renewable and biodegradable nature (Aziz & Ansell, 2004). Among all the natural fibre reinforcing materials, jute appears to be a promising material because it is relatively inexpensive and commercially available in the required form. However, its mechanical and physical properties are highly inconsistent and depend on geographic origin, climatic growth conditions and processing techniques (Gowda, Naidu & Chhaya, 1999). Jute is one of the most important natural fibres, and is produced in India, Bangladesh, Thailand, Vietnam and other countries. It contains 56-64% cellulose, 29-25% hemicelluloses, 11-14% lignin and a small proportion of fats, pectin, ash and waxes (Murphy, 1998).
Materials and Methods
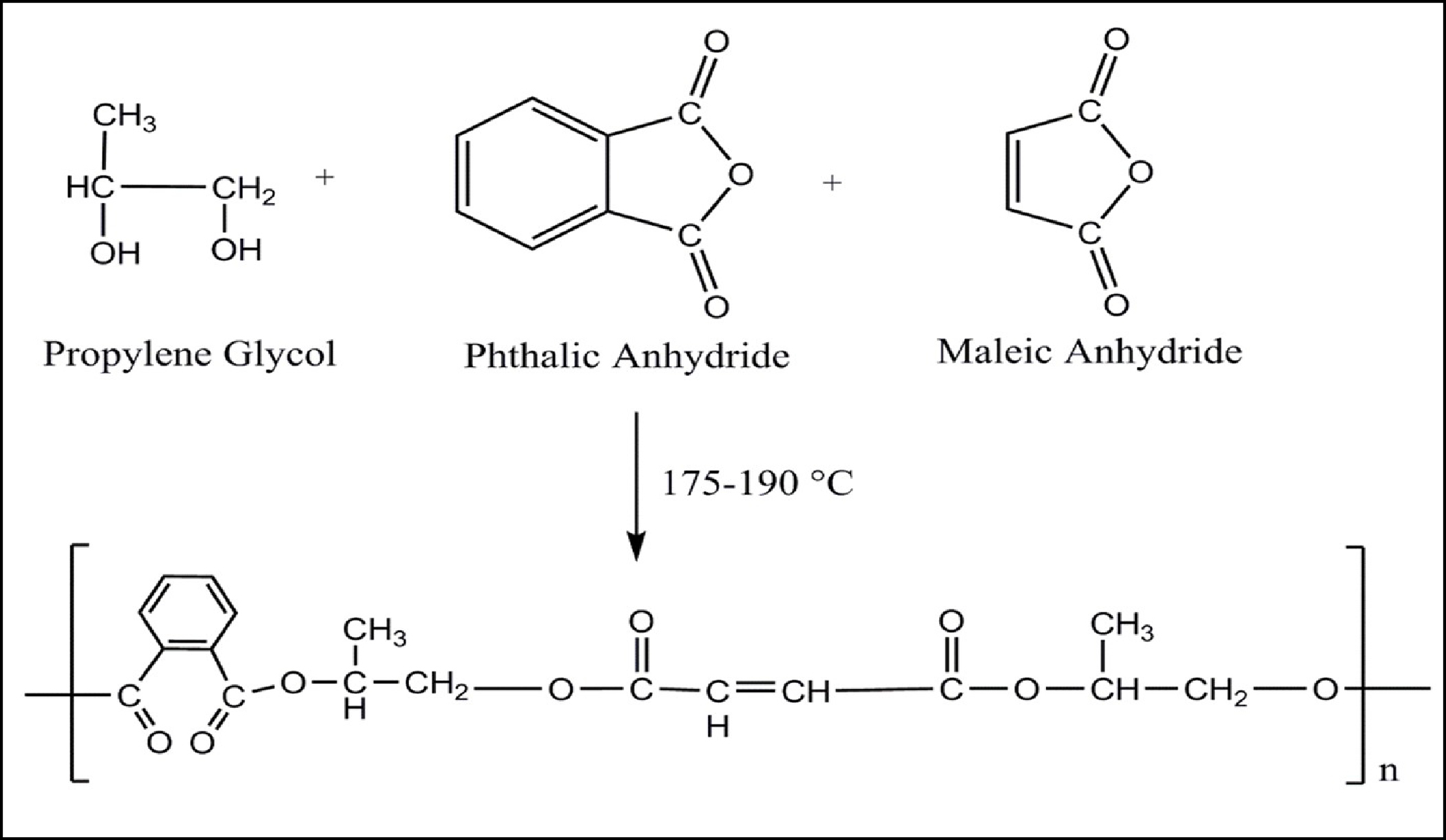
Jute fibre, phthalic anhydride, maleic anhydride, propylene glycol and p-toluene sulfonic acid were obtained from Chiti -Chem Corp. Ltd, Vadodara. All the chemicals used were of analytical grade.
Synthesis of Polyester Resin
A mixture of propylene glycol (PG), phthalic anhydride, maleic anhydride, p-toluene sulfonic acid and xylene as distillation solvent was charged in a four-neck reaction kettle equipped with stirrer, thermometer, nitrogen-gas introducing tube, Dean & Stark apparatus, water condenser. The mixture was mechanically stirred and heated at 140-2000C under nitrogen gas stream; and esterification was carried out while removing water formed due to the reaction in the system. Heating is continued at 140-2000C until an acid number of 25-30 was reached. The xylene was completely distilled out and reaction product was allowed to cool.
Scheme-1: General Reaction mechanism for polyester resin (Unsaturated Polyester Resin-UPR).
A mixture of propylene glycol (PG), phthalic anhydride, maleic anhydride, p-toluene sulfonic acid and xylene.
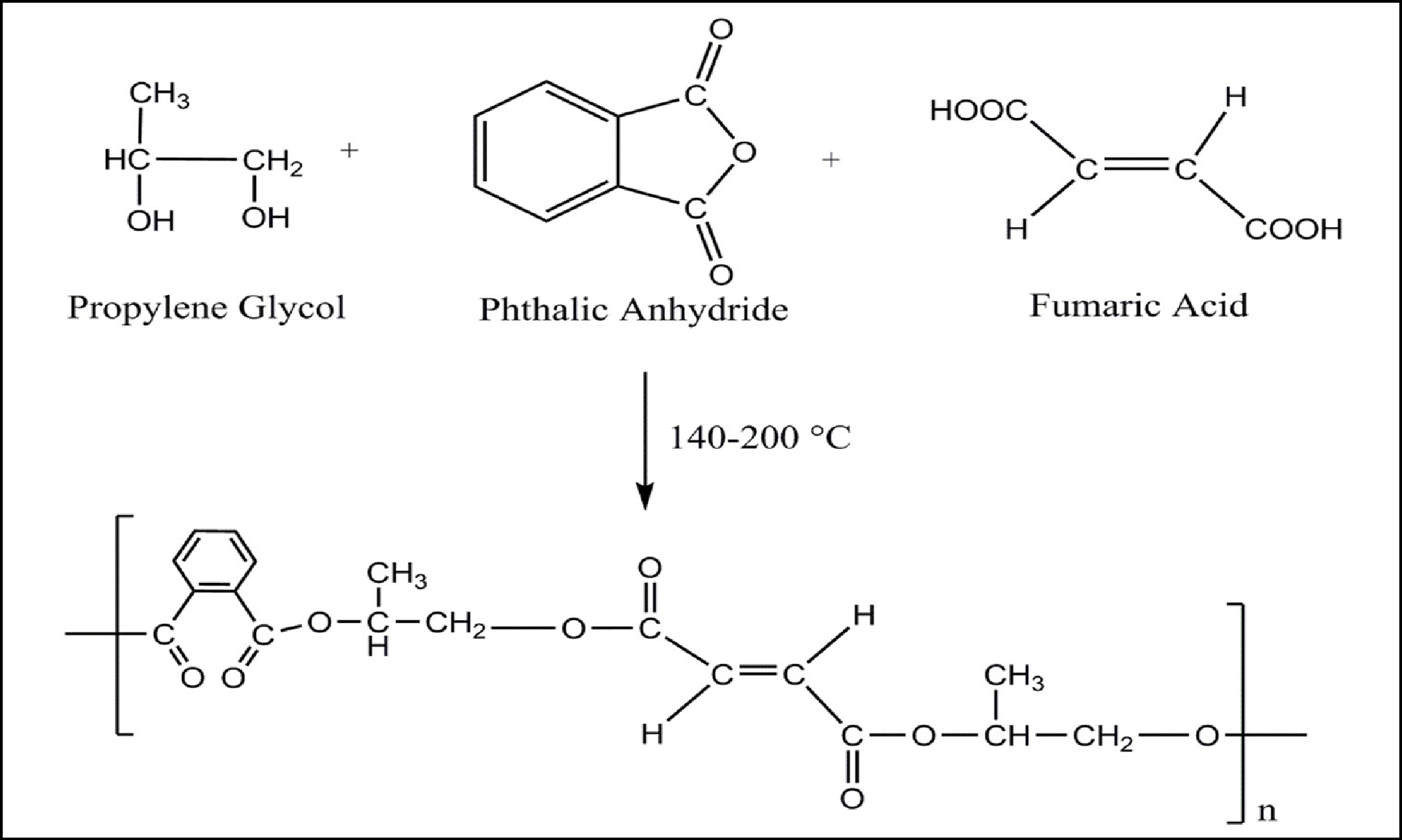
Synthesis of fumaric acid modified unsaturated polyester resin (FAMUPR).
A mixture of propylene glycol (PG), phthalic anhydride, fumaric acid, p-toluene sulfonic acid and xylene.
Scheme-2: Reaction mechanism for fumaric acid modified unsaturated polyester resin.
Composite Fabrication
Two basic methods are generally used for the fabrication of composites, which are known as wet and dry lay-up lamination. Wet lay-up method implies the use of liquid resins to
impregnate the matrix material either before or after it has been laid in place. In case of conventional dry-up method, the reinforcement is impregnated with a solution of resin in appropriate solvent; the solvent is evaporated or flushed off and the result is dry resin impregnated sheet, which is used to prepare composites (Bartenev, Povarova & Kargin, 1964). Laminating is normally carried out manually by brush, roller or squeezer application to glass fibre (Kumar, Kumar & Subbaiah, 2020; Patel et al., 2013, 2014; Patel, 2020).
Characterization of resin & composite
Reaction was monitored by continuous determination of the reaction mass. Completion of reaction was observed with acid value, FTIR and data was compared with a standard (Table 2). The obtained composites were characterized by TGA & DTA (Fig. 2 & 3).
Table 1. Composition of composites for fumaric acid modified unsaturated polyester resin (FAMUPR).
|
Sample
|
Jute
(40% of total wt.)
in gms
|
Polyester resin
(60% of total wt.)
in gms
|
Total Weight
in gms
|
Curing agent
(2% of total wt.)
in gms
|
Curing Temp.
in oC
|
Curing time
in hrs.
|
|
FAMUPR.1
|
35.50
|
53.25
|
88.75
|
1.77
|
100-110
|
2
|
|
FAMUPR.2
|
35.08
|
52.56
|
87.66
|
1.75
|
100-110
|
2
|
|
FAMUPR.3
|
34.40
|
51.60
|
86.00
|
1.72
|
100-110
|
2
|
Table 2. Infrared spectra of fumaric acid modified unsaturated polyester resin (FAMUPR.1).
|
No.
|
Group
|
IR Characteristics (cm-1)
|
IR for S-21
|
|
A
|
Esters α,β - unsaturated >C=O stretching
|
1730-1715
|
1729.00
|
|
B
|
C-H Stretching in aromatic
|
~3030
|
3000.25
|
|
C
|
C-C multiple bond stretching in Alkene
|
1620-1680
|
1650.50
|
|
D
|
C-C multiple bend stretching in aromatic
|
~1600
|
1602.58
|
|
E
|
Hydrocarbon Alkane –CH2-
|
1445-1485
|
1450.45
|
|
F
|
Alcohols O-H bond stretching
|
1260-1350
|
1282.91
|
|
G
|
C-H stretching in aromatic ring (O- disubstituted)
|
735-770
|
750.40
|
Infrared (IR) spectral study of polymers provide useful information about their structural features as it gives the idea about the nature of functional groups and the skeletal structure of the polymers. In suitable cases, the IR spectral study has been proved useful in understanding the course of a polymerization reaction (Hummel, 1966). There are several reports about the IR spectral study of the polymers in the detection of crystallinity in the sample and the estimation of stereo regularity in the polymer chains (Richards, 1951). IR spectroscopy is widely used for structural elucidation of the polymers (Hummel, 1966).
A Nicolet Impact 400D FT-IR Spectrophotometer was employed for the measurements. The spectrum was run by applying resin sample on KBr cells covering the range of frequencies from 400-4000 cm-1. The IR spectra of unsaturated polyester resins are given in Fig. 1. All the IR spectra are similar in general shape, however some variations in characteristic absorption peaks are observed. The important bands observed and their possible assignments are described in Table 2.
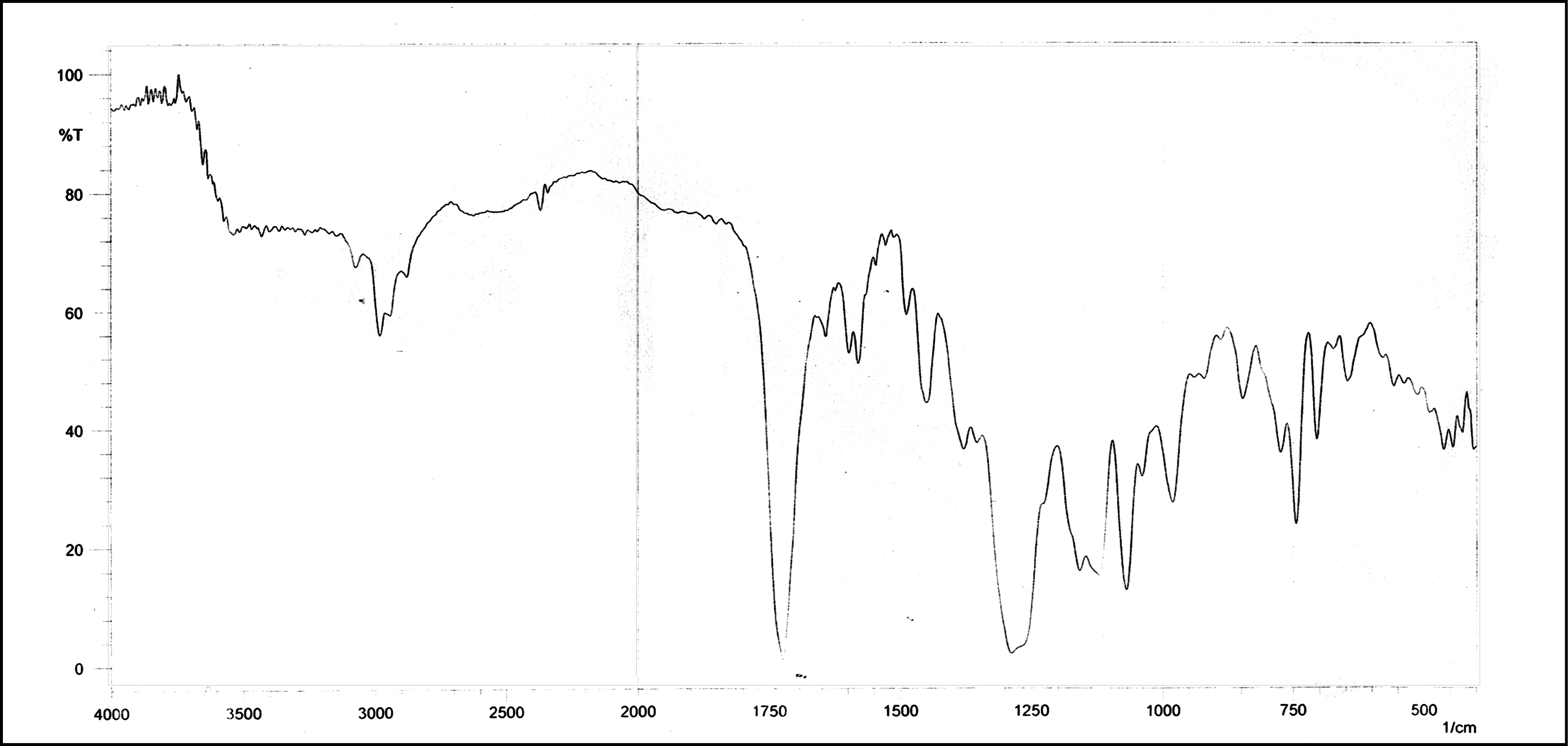
Figure 1. Infrared spectra of fumaric acid modified unsaturated polyester resin (FAMUPR.1).
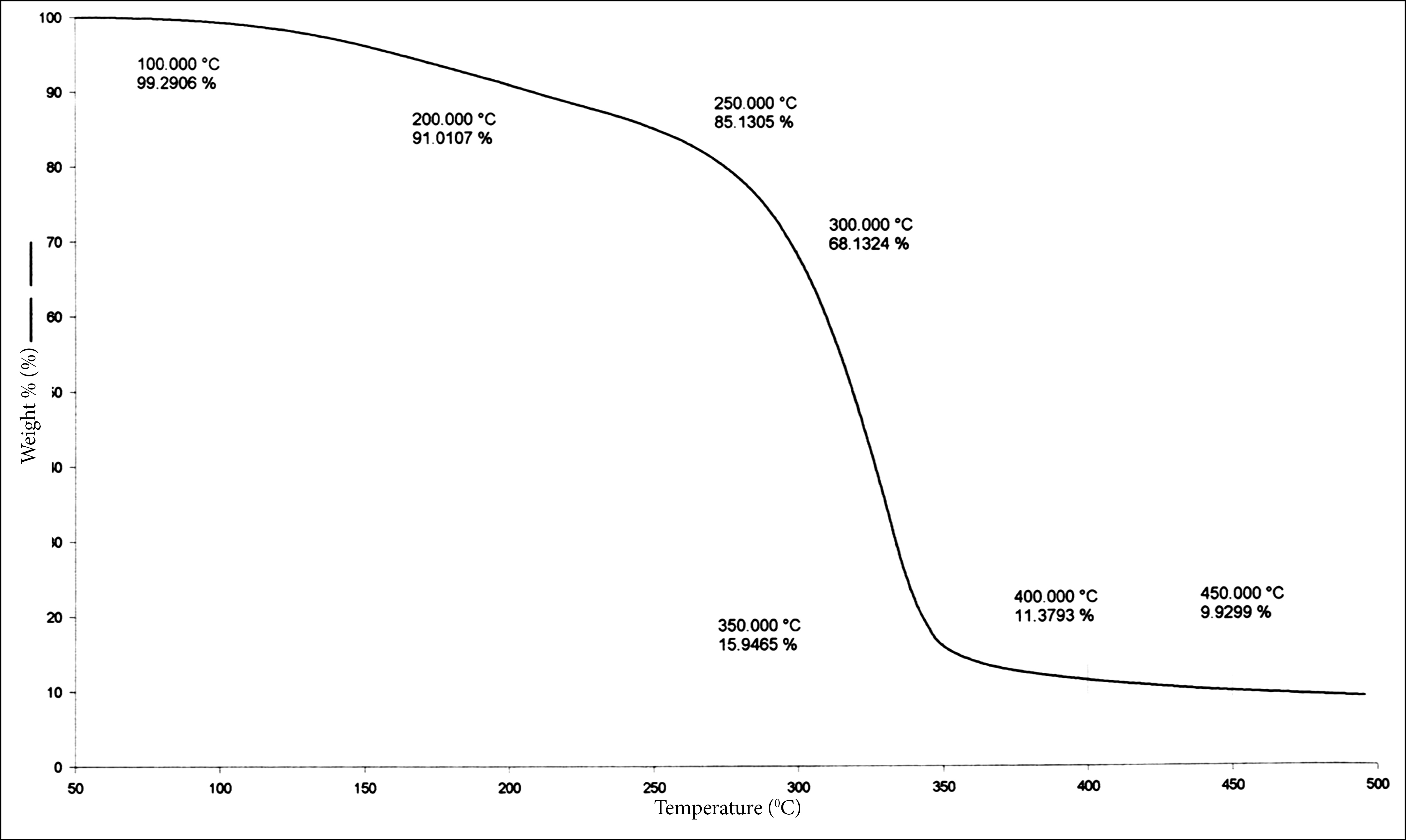
Figure 2. TGA of fumaric acid modified unsaturated polyester resin (FAMUPR.1).
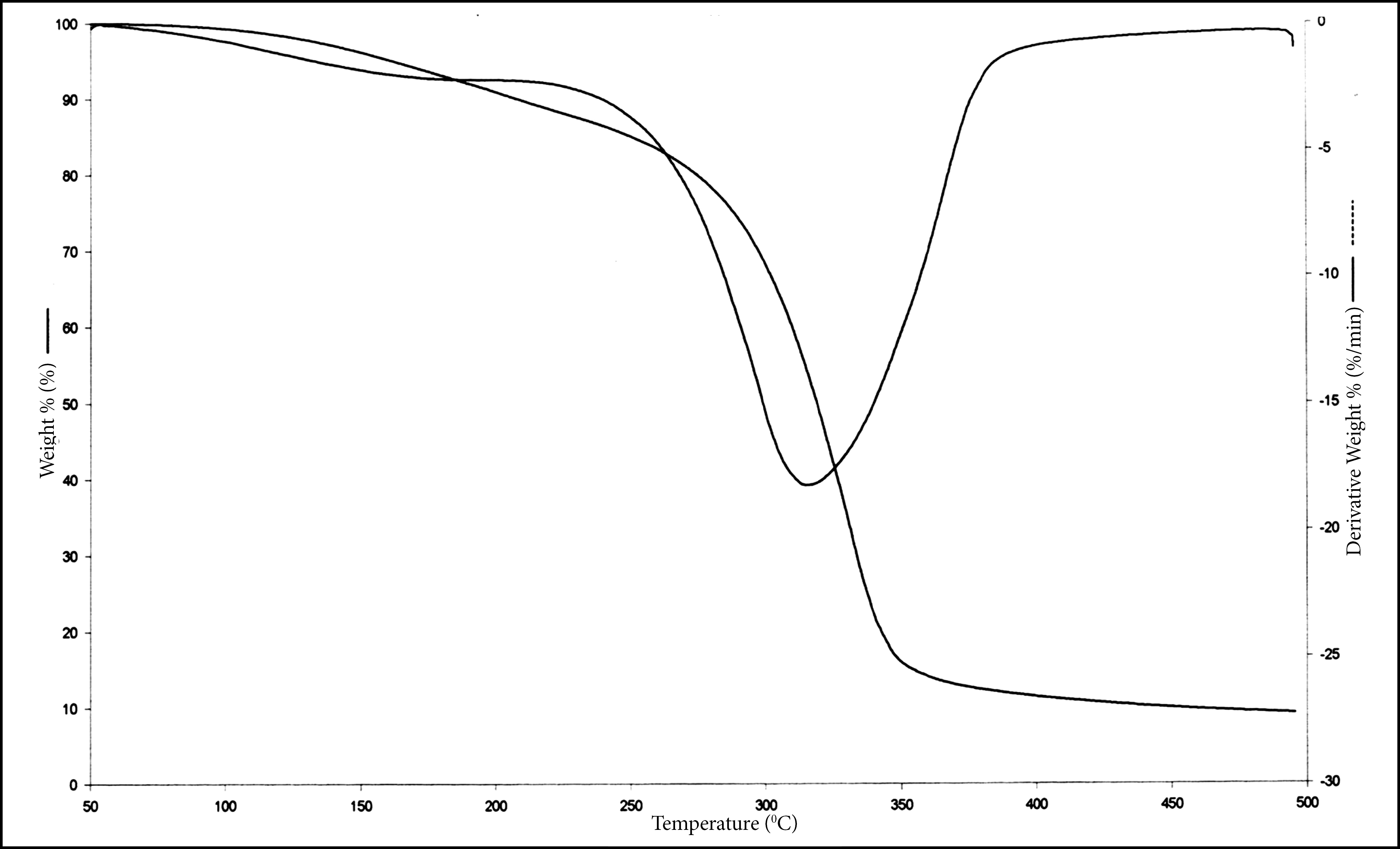
Figure 3. DTA scan of fumaric acid modified unsaturated polyester resin (FAMUPR.1).
Results and Discussion from IR
The IR spectra of modified polyester resins are as shown in Fig. 1 The data regarding the IR spectral characteristics presented in Table 2 reveal small variations in the location of the peaks due to absorptions by functional groups like –CH2, C=C, and C=O; and are observed depending upon the structure of the diols/anhydrides/acids.
For modified polyester resins, a strong absorption band at 750.40 cm-1 can be attributed to -C-H stretching in aromatic ring (O-disubstituted). Spectrum absorption band at 1729 cm-1 and 300.25 cm-1 confirms the presence of α,β-unsaturated >C=O bond in ester linkage and C-H stretching in aromatic respectively. Absorption peak appearing at 1650.50 cm-1 and 1602.58 cm-1 was C-C multiple bond stretching in alkene and C-C multiple bond stretching in aromatic. Alkane -CH2- and O-H bond stretching was confirmed by the presence of band at 1450.45 cm-1 and 1282.91 cm-1 respectively.
The TGA data in Table 3 indicate that the thermal stability increases when maleic anhydride is replaced by fumaric acid up to 300oC. FAMUPR.1 is thermally more stable than regular polyester resin. After 300oC thermal stability is approximately similar for both regular polyesters resin and modified polyester resins.
Mechanical Property
Flexural properties
It is one of the most important mechanical properties of interest for any comparison of rigid materials or modulus of rupture. Flexural properties are useful for quality control and specific purpose. In addition, they serve to classify material with respect to their bending strength and stiffness characteristics. The stress-strain behavior of polymers is of interest to a designer as well as a polymer manufacture.
Impact properties
The impact properties of the polymeric materials are directly related to the overall toughness of the material. Higher the impact strength of the material, higher is the toughness and vice versa. Impact resistance is the ability of a material to resist breaking under a shock loading or the ability to resist fracture under stress applied at high speed.
Izod impact test
The objective of the Izod impact test is to measure the relative susceptibility of a standard test specimen to the pendulum type impact load. The results are expressed in terms of kinetic energy consumed by the pendulum in order to break the specimen. The energy lost through the friction and vibration of the apparatus is minimum and common for all practical purposes and generally neglected.
Table 3. TGA data.
|
Sample
|
% weight loss at various temperature (OC) from TGA
|
|
100
|
200
|
250
|
300
|
350
|
400
|
450
|
|
UPR.S1
|
0.38
|
7.68
|
24.20
|
47.51
|
84.87
|
86.66
|
-
|
|
FAMUPR.1
|
0.71
|
8.99
|
14.87
|
31.87
|
84.05
|
88.62
|
90.07
|
|
FAMUPR.2
|
1.13
|
11.42
|
17.16
|
36.08
|
84.65
|
90.36
|
91.50
|
|
FAMUPR.3
|
1.58
|
15.27
|
22.57
|
40.86
|
87.25
|
91.25
|
-
|
Table 4. Flexural Strength & Flexural Modulus.
|
Sample
|
Flexural Strength
kg/cm2
|
Flexural Modulus
kg/cm2
|
Izod Impact Strength
J/cm2
|
Rockwell Hardness
“M” SCALE
|
|
FAMUPR.1
|
2065.27
|
26528.68
|
2.64
|
109
|
|
FAMUPR.2
|
1824.12
|
23416.43
|
1.92
|
135
|
|
FAMUPR.3
|
1802.12
|
20008.00
|
1.25
|
146
|
Conclusion
Unsaturated polyester resin (UPR) is being compared with modified polyester resin. The flexural strength and flexural modulus of fumaric acid modified unsaturated polyester resin, FAMUPR.1 to FAMUPR.3 decreases but is higher than unsaturated polyester resin (UPR). The Rockwell Hardness of fumaric acid modified unsaturated polyester resin (FAMUPR) is higher than unsaturated polyester resin (UPR).
The impact strength of all modified polyester resin decreases if moles of fumaric acid increases but the impact strength of all modified polyester resin (FAMUPR) is higher than the regular unsaturated polyester resin (UPR), except FAMUPR.3.




